| | Parameters invalid or missing | e.g. "parameters error"Please check your input parameters.
Some required parameters might be missed.
e.g. "splitBcPos error, expected 1_24 or 2_25"
Please check your input CID position is either 1_24 or 2_25. |
| | Please check the input file exists and has the correct access permission. |
| | e.g. "only support .bin or .h5 file."
Please check your input file is in the correct file format. |
| | e.g. "cannot write to file, /path/to/file"
Please check your output directory path is an existing path. |
| | Please check Appendix C or contact FAS/FBS for help. |
| | Please check Appendix C or contact FAS/FBS for help. |
| | Parameters invalid or missing | Please check your input parameters. Some required parameters might be missed. |
| | e.g. "cannot open such file, /path/to/file"
Please check the input file exists and has the correct access permission. |
| Failed to parser the file | Please check your input file is in the correct file format. |
| | Please check Appendix C or contact FAS/FBS for help. |
| | Please check Appendix C or contact FAS/FBS for help. |
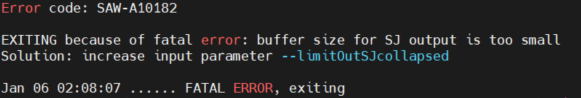
| | Parameters invalid or missing | e.g. "EXITING: FATAL INPUT ERROR: unrecognized parameter name "outSAMattribute" in input "Command-Line-Initial""
Please check the spelling of your argument and parameters.
e.g. "please check the umi position and length"
Please check the length of the reads in FQ1 are consistent with the parameters set for barcode length and umi length. For example, the length of the sum of barcode and umi set in bcPara file is 35 bp, but one of the reads in FQ1 is 30 bp, then you will see A10101 error code. Please check the parameters set in the bcPara file are consistent with your FQs. |
| | e.g. "Error, cannot open the file which be expected in gz or ascii format"
Please check the file permission and file format.
e.g. "barcodePositionMapFile does not exists: /path/to/mask"
Please check the mask file exists and has the correct access permission. |
| | e.g. "sequence and quality have different length"
Please check the completeness of the reads in FQ. This issue may arise if the FQs are in incorrect format or the file was incompletely written or transferred. |
| Invalid data or data exception | Error data. Please check the file format and content. |
| | This error arose if "_STARtmp" directory failed to be deleted. Please check whether the program has completely finished according to the *.Log.progress.out or *_barcodeMap.stat file. |
| | Please contact FAS/FBS for help. |
| Failure on APIs of system and libraries | Please contact FAS/FBS for help. |
| | Please contact FAS/FBS for help. |
| | Please contact FAS/FBS for help. |
| | This error occurs when you run out of RAM. You may need to kill some jobs that use the same RAM or upgrade your hardware to get more RAM resources for your job. |
| | This error occurs when you store too many files on your hard disk. Please remove some files to free disk space. |
| | Please check your input parameters. Some required parameters might be missed. |
| CID comparison rate is too low | This error usually arises because the CID information of the input FQs is not the same as the CID in the input mask file. Please use the correct SN-FQ pairs for mapping. |
| Fail to create the index for the BAM file | Please contact FAS/FBS for help. |
| Fail to load indexed reference | Please check the existence, access permission, and completeness for the indexed reference. |
| | | Please check your input parameters. Some required parameters might be missed. |
| | Please check the input file exists and has the correct access permission. |
| | Please check your input BAM header is in the correct file format. |
| | This error occurs when you run out of RAM. You may need to kill some jobs that use the same RAM or upgrade your hardware to get more RAM resources for your job. |
| | Please contact FAS/FBS for help. |
| | Please check your input parameters. Some required parameters might be missed. |
| | Please check the file that exists and has the correct access permission. |
| | Please check your input file has a valid column number. |
| | | Please check your input parameters. Some required parameters might be missed. |
| | Please check the input file exists and has the correct access permission. |
| | Please check your mask file is in the correct file format. Only support .h5/.bin mask file. |
| Fail to create output file | Fail to create output file. Please check your writing permission of the output directory. |
| | Fail to open input TXT file. Please check the file that exists and have the correct access permission. |
| | Record coordinates exceed the expected range. Please check the input file is the output of mapping. |
| | Please check whether the range of the coordinates is too large, or you have run out of RAM. You may need to kill some jobs that use the same RAM or upgrade your hardware to get more RAM resources for your job. |
| | | Please check your input parameters. Some required parameters might be missed. |
| | Please check the input file that exists and has the correct access permission. Or, please check whether a stitched panoramic TIFF (.tif or .tiff) image exists in the TAR.GZ. |
| | Please check your input file is in the correct file format. The -v input gene expression matrix should be either a *tsv, barcode_gene_exp.txt, *.gem.gz, or *raw.gef. |
| Invalid data or data exception | Error data. Please check the file content. This error may arise because the -v input file is empty, the CZI file in TAR.GZ is invalid, or the QCPassFlag in IPR is 0. |
| Tissue segmentation error | Abnormal tissue segmentation reference score in image preprocessing. |
| | "Stitch/BGIStitch/StitchedGlobalLoc", does not exist. |
| | Please check the uncompressed image folder has tiled images. |
| | | Please check your imageTools merge input parameters. Some required parameters might be missed. |
| | Please check the imageTools merge input file that exists and has the correct access permission. |
| | imageTools merge inputs of less than two images or more than three images. |
| | Please check the imageTools merge input TIFF sizes are the same. Since the merge function is used for evaluating segmentation results, the input images are supposed to be the same in size and position (tissue position in the whole image). |
| | Please check your imageTools overlay input parameters. Some required parameters might be missed. |
| | Please check the imageTools overlay input file that exists and has the correct access permission. |
| Invalid data or data exception | Error data. Please check the file content. This error may arise because the -c input IPR file does not include Stitch/TransformTemplate or Register/MatrixTemplate information |
| | Please check your imageTools img2rpi input parameters. Some required parameters might be missed. |
| | Please check the imageTools img2rpi input file that exists and has the correct access permission. |
| | imageTools img2rpi input -i and -g have different length. These two inputs are supposed to be paired. |
| | Please check your imageTools ipr2img input parameters. Some required parameters might be missed. |
| | Please check the imageTools ipr2imginput file exists and has the correct access permission. Or, please check whether a stitched panoramic TIFF (.tif or .tiff) image exists in the TAR.GZ. |
| | Please check your imageTools ipr2img input file is in the imageQC/imageStudio output TAR.GZ format. |
| Invalid data or data exception | Error data. Please check the imageTools ipr2img input IPR file content.
This error may arise because the CZI file in TAR.GZ is invalid, or the image has not either automatically or manually registered with the expression matrix. The second circumstance can be confirmed from IPR by checking whether the StereoResepSwitch/register is TRUE (has not performed automatic registration) or the ManualState/register is FALSE (has not performed manual registration). The third possible reason is the StereoResepSwitch/tissueseg is TRUE, therefore cannot output cell segmentation result. |
| | Please check the shape of registered tissue segmentation images stored in the imageTools ipr2img input IPR are the same. Or please contact FAS/FBS for help. |
| | | Please check your input parameters. Some required parameters might be missed |
| | Please check the file that exists and has the correct access permission. |
| | Please check your input file is in the correct file format. |
| Fail to create output file | Fail to create output file. Please check your writing permission of the output directory. |
| | Fail to write a TIFF file. |
| | Please check the H5 file attributes. |
| | Please check the H5 file dataset. |
| | | Please check your input parameters. Some required parameters might be missed |
| | Please check the file that exists and has the correct access permission. |
| | The file does not contain correct information. Please check the file format. |
| | Please check your output GEF version. Your input GEF version might be too old. |
| | e.g. "Please call freeRestriction first, or call restrictRegion function before restrictGene."
This error arose because the invocation flow order was messed up. Please modify your invocation flow as prompted. |
| Invalid data or data exception | Error data. Please check the file content. |
| | Failed to read the file. Please check whether the file is damaged. |
| | Please check the TIFF mask size is consistent with the size of expression matrix. Since the mask has been registered with the expression matrix, their sizes are supposed to be the same. |
| Fail to create output file | Fail to create output H5 file. Please check your writing permission of the output directory or contact FAS/FBS for help. |
| | This error occurs when you run out of RAM. You may need to kill some jobs that use the same RAM or upgrade your hardware to get more RAM resources for your job. |
| Dimensions of gene expression matrix did not match | Please contact FAS/FBS for help. |
| | | e.g. "-i or --gef_file is missing"
Please check your input parameters. Some required parameters might be missed. |
| | e.g. “cannot access /path/to/file: No such file or directory.”
Please check the file that exists and has the correct access permission. |
| | e.g. "The bin size is out of range, please check the range of gef binsize is in [1,10,20,50,100,200,500]."
Please reset the bin size as prompted.
e.g. "Gene number less than 3000, please check your gef file"
Please check the content of your GEF file, and make sure there are at least 3000 genes for clustering. |
| | | e.g. "-i or --gef_file is missing"
Please check your input parameters. Some required parameters might be missed. |
| | e.g. “cannot access /path/to/file: No such file or directory.”
Please check the file that exists and has the correct access permission. |
| | e.g. "The bin size is out of range, please check the range of gef binsize is in [1,10,20,50,100,200,500]."
Please reset the bin size as prompted.
e.g. "Gene number less than 3000, please check your gef file"
Please check the content of your GEF file, and make sure there are at least 3000 genes for clustering. |
| | | e.g. "-i is missing."
Please check your input parameters. Some required parameters might be missed. |
| | Please check the file that exists and has the correct access permission. |
| | Invalid GEF file. Please check the file format. |
| Invalid data or data exception | e.g. "no data left after filter by coordinates."
Please check the file content. |
| Invalid data or data exception | e.g. "total map reads is 0, please check file format from --bcstat"
Please check the file content of the input file as prompted. |
| | e.g. "map reads less than annotated reads."
Please check the input mapping statistical report and the count statistical report are from the same analysis. |
| | Please contact FAS/FBS for help. PATH environment may not have python3. |
| Fail to create output file | Fail to generate saturation file, please contact FAS/FBS for help. |
| | | e.g. "-m or --barcodeMapStat is missing."
Please check your input parameter as prompted. Some required parameters might be missed. |
| | e.g. "cannot access *: No such file or directory."
Please check the file that exists and has the correct access permission. |
| | JSON file format error. This error may arise because the input statistics files were not generated in the same SAW version as report. Or, the input mapping file prefix can not be parsed. Please contact FAS/FBS for help. |
| Invalid data or data exception | e.g."information loss: fail to find 'bin_[size]' or 'ssDNA' in '*.rpi'."
Please check the file content. |
| Fail to create output file | Fail to create output file. Please check your writing permission of the output directory. |
| | | Please check your input parameters. Some required parameters might be missed |
| | Please check the file that exists and has the correct access permission. Or, please check whether the pre-registered image fov_stitched_transformed.tif exists in the input directory. |
| | Please check your input file is in the correct file format. The -v input gene expression matrix should be either a *tsv, barcode_gene_exp.txt, *.gem.gz, or *raw.gef. |
| Invalid data or data exception | Error data. Please check the file content. This error may arise because the -v input file is empty. The second possible reason is that the gene expression matrix information in the IPR Register module (MatrixShape, Xstart, Ystart) does not match with the input GEF file (minX, minY, maxX, maxY), because the manual registration has to be processed on the identical matrix. |