All
Products
Resources
News
FAQ
Search
09/08/2022
Cervical cancer is the fourth most dangerous cancer for women's health worldwide. According to statistics, nearly 570,000 new cases of cervical cancer were reported worldwide in 2018.
The World Health Organization (WHO) officially published “the Global Strategy to Accelerate the Elimination of Cervical Cancer” in 2020, committing 194 countries worldwide to eliminate a cancer for the first time. Effective management and treatment of patients with cervical precancer or cancer will remain one of the priorities of the health care system for decades to come.
Recently, the international journal Advanced Science published the research results of a collaboration between BGI Research, Tongji Hospital of Huazhong University of Science and Technology School of Medicine, and Chongqing Southwest Hospital, which used Stereo-seq to identify a class of cancer-promoting cancer-associated fibroblasts (myCAFs) in cervical squamous carcinoma clinical tissues for the first time. These cells are expected to be potential targets for the treatment of advanced cervical squamous carcinoma, contributing to the development of new prognostic and therapeutic approaches and providing scientific guidance for the development of clinical drugs for cervical squamous carcinoma.
Screenshot of Advanced Science official website
Cervical cancer is mainly caused by persistent infection with human papilloma virus (HPV), the majority of which are HPV16-positive squamous cervical cancers. Patients with primary cervical cancer can have a good prognosis with hysterectomy, but the 5-year overall survival rate for patients with advanced cervical cancer is not ideal. Currently, chemotherapy and radiotherapy remain the main palliative treatments for metastatic or recurrent cervical cancer.
The Global Strategy to Accelerate Cervical Cancer Elimination outlines three key measures: vaccination, screening and treatment. Among these, the treatment target to be achieved by 2030 in all countries or regions is that 90% of women diagnosed with cervical disease receive treatment.
In recent years, immunotherapy has brought new hope for the treatment of advanced cancer, but unfortunately, the overall response rate of advanced cancer to immunotherapy is low, with only less than 30% of patients receiving effective treatment. Therefore, further understanding of the immune status of cervical squamous carcinoma, especially the immunosuppressive state in the tumor microenvironment, will help us better explain this phenomenon, improve the treatment strategy for advanced cervical cancer, and help eliminate cervical cancer.
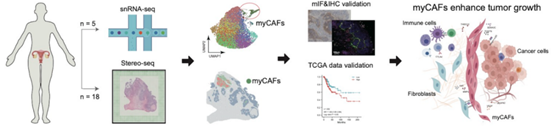
This research team collected cervical lesion tissues from 20 women and combined Stereo-seq, single cell and immunohistochemistry to resolve the microenvironment of cervical squamous carcinoma. The research team identified cancer-associated fibroblasts in clinical tissues of cervical squamous carcinoma and explored their function in promoting cancer growth.
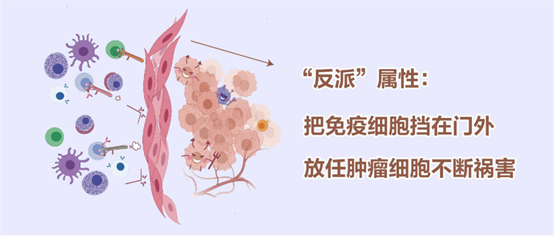
Cancer-associated fibroblasts (myCAFs) can be simply understood as the "accomplices" of cervical cancer. According to the Stereo-seq data, myCAFs are able to accumulate around tumors, forming a physical barrier that prevents immune cells from entering the cancer site and helps tumor cells fight against the body's immune system; at the same time, they secrete cytokines to promote tumor cell proliferation, angiogenesis, and remodeling of the extracellular matrix, thus promoting the growth and metastasis of cancer cells.
For relevant validation, the research team obtained data from 252 cases of cervical squamous carcinoma through the TCGA public database, and analysis based on transcriptomic data showed that patients with cervical squamous carcinoma had lower survival rates when myCAFs signature genes were more highly expressed. In historical pathology samples, about 20% of the samples had high expression of marker proteins of myCAFs in the extracellular stroma, and the higher the expression level, the larger the size of the cancer and the higher the degree of lymph node metastasis. This evidence suggests that myCAFs may promote the growth and metastasis of cancer foci.
When applying immunotherapy, is it possible to target myCAFs to inhibit their function for allowing patients to get better treatment results?
To further investigate whether myCAFs affect sensitivity to immunotherapy in patients with cervical squamous carcinoma, the team used publicly available data to correlate myCAFs signature gene expression levels with clinical assessments of immunotherapy sensitivity metrics. The findings all indicated that myCAFs positively correlated with tolerance to immunotherapy in cervical squamous carcinoma.
The cancer-promoting properties of myCAFs are expected to provide new targets for the prognosis and treatment of advanced cervical cancer. Simultaneous killing of myCAFs or inhibition of their functions when using immune checkpoint blockade therapy may yield better efficacy. The function and clinical value of the specific genes of myCAFs in cancer progression also deserve further investigation. In addition, the study also revealed the expression of HPV genes in cancer foci and the association between tumor metabolism and the strength of immune response. Overall, this study provides high-resolution observational data on the virological, cytological and immunological features of cervical squamous carcinoma, which will contribute to the development of new prognostic and therapeutic approaches.