All
Products
Resources
News
FAQ
Search
05/09/2025 Yahui Li
Hepatocellular carcinoma (HCC), a major form of primary liver cancer, strikes nearly 1 million people annually and ranks among the leading causes of cancer-related deaths worldwide. Recently, immune checkpoint blockade therapy (ICB), a type of immunotherapy, has revolutionized HCC treatment and become a crucial component of the first-line therapy for advanced HCC. Tertiary lymphoid structures (TLS) are ectopic lymphoid aggregates that have been shown to be associated with improved responses to immunotherapy and are drawing increasing attention in new cancer treatments. [2-3]
A recent Cancer Cell study [1] employed spatial transcriptomics to investigate TLS in HCC clinical samples, uncovering TLS distinct developmental states and implicating tumor-derived tryptophan metabolism as a key regulator of TLS maturation. Combining with single-cell RNA-seq (scRNA-seq), single-nucleus ATAC-seq (snATAC-seq) and in vivo experiments, the authors also mechanistically validated their findings. This data-driven study not only reveals novel therapeutic targets for anti-tumor immunity, but also provides a translatable framework to dissect immune regulation in tumor microenvironments across cancers.
It is normal that you may not all be cancer biology experts. The below outlined key concepts will help you contextualize this study:
Tertiary Lymphoid Structures (TLS)
TLS are transient ectopic lymphoid aggregates that form in non-lymphoid tissues. They are closely associated with improved prognosis and enhanced response to immunotherapy in various cancers.
Immune Checkpoint Blockade Therapy (ICB)
ICB is a type of cancer immunotherapy that activates T-cell antitumor responses by inhibiting immune checkpoint molecules (e.g., PD-1/PD-L1, CTLA-4).
Naive B cells vs. Germinal Center (GC) B cells
Naive B cells are mature but antigen-inexperienced B cells that have exited the bone marrow without encountering their cognate antigen.
GC-B cells are antigen-experienced B cells that undergo rapid proliferation and affinity maturation within GCs of lymphoid follicles after antigen exposure. Mature TLS contain GCs, which are critical for B-cell development and function.
Tumor Microenvironment (TME)
TME is the ecosystem surrounding a tumor, including immune cells, blood vessels, and signaling molecules, which can either suppress or promote cancer growth.
Metabolic Reprogramming
Metabolic reprogramming refers to how cancer cells alter their metabolism to fuel rapid growth and survival.
![]()
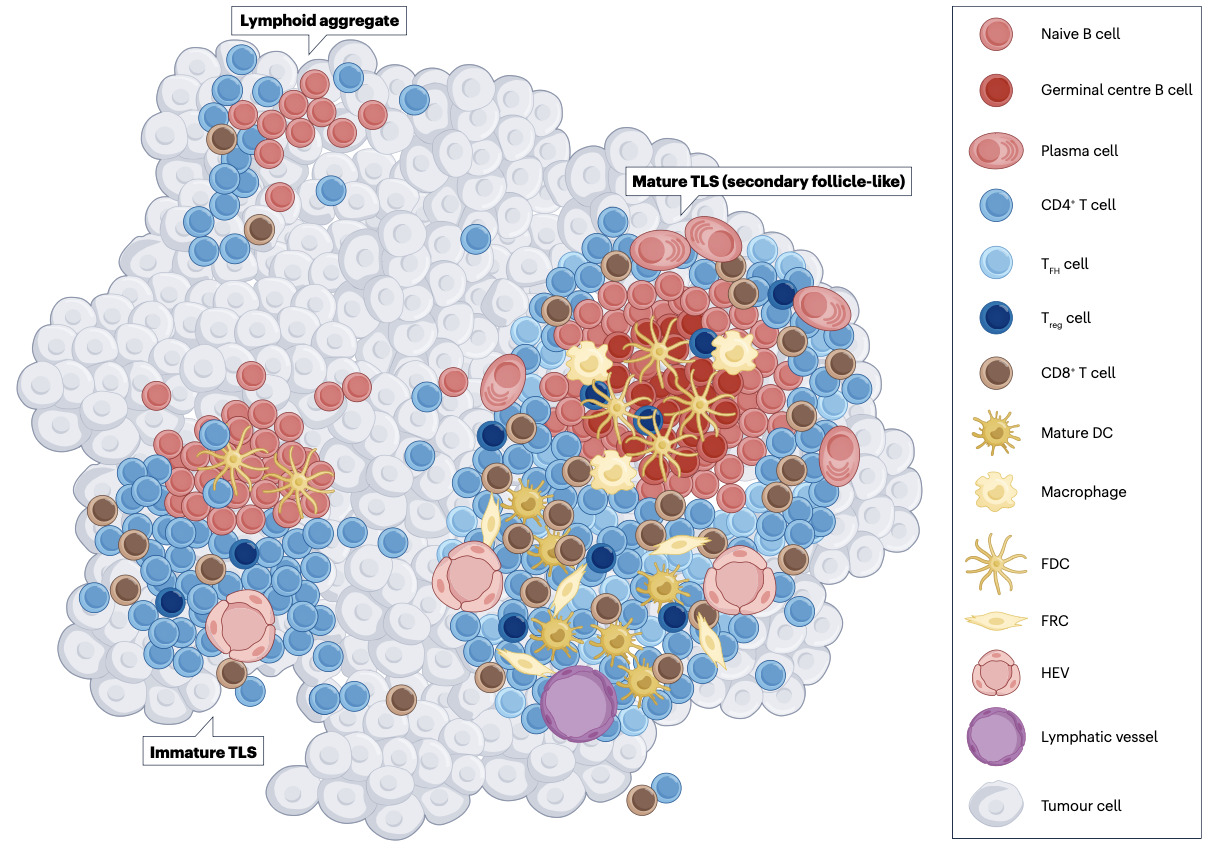
Developmental stages of tertiary lymphoid structures (TLS): From lymphoid aggregates to immature and mature TLS. From Singal et al. [3]
In this study, the researchers used Stereo-seq to profile fresh-frozen tissue specimens from 22 treatment-naive HCC patients and 7 patients who received anti-PD-1 immunotherapy. This approach allowed them to capture spatial transcriptomic data from expansive tissue sections at near-cellular resolution, providing a detailed map of the tumor microenvironment (TME).
The data-driven logic of the study involved several key steps. First, the researchers developed a computational strategy to identify TLS based on their spatial transcriptional properties. They segmented tissue sections into distinct spatial domains and identified regions with pronounced expression of TLS-related genes. This method allowed them to accurately detect 838 TLSs across tissue sections from the treatment-naive cohort.
250905.png)
Figure 1A-B from Tang et al. (2025) [1] illustrates the spatial transcriptomics experimental design and key steps of the proposed TLS identification pipeline.
Next, the study aimed to decode the developmental states of TLS. Researchers developed a convolutional neural network model to classify immature TLS into two groups: conforming and deviating, based on their conformity to a benchmark differentiation trajectory from naive B cells to germinal center B (GC-B) cells. This classification revealed distinct characteristics in antitumor immunity, with conforming TLS showing a niche function similar to mature TLS, while deviating TLS did not.
250905.png)
Figure 2C from Tang et al. (2025) [1] shows the classification of TLS developmental states based on transcriptional similarity of naive B and GC-B cells along differentiation trajectories.
The team also evaluated the clinical prognostic value of conforming TLS. They established a 10-gene signature that accurately distinguishes between conforming and deviating TLS subtypes. This signature robustly predicted recurrence-free survival and strongly correlated with ICB response. These findings position TLS subtypes as prognostic biomarkers for survival outcomes and predictive for immunotherapy response.
How do some TLS stay stuck in an immature state? By tracking gene activity of naive B and GC-B cells along the maturation trajectory, the team spotted a clear clue: a cluster of genes are steadily ramp up from mature to conforming to deviating TLS that highlight a few tryptophan-metabolism genes (i.e. TDO2, IDO2), and the tryptophan metabolism is also highlighted as an enriched pathway for this cluster of genes.
250905.png)
Figure 4A-C from Tang et al. (2025) [1] demonstrate significant enrichment of tryptophan-metabolism related genes and pathway among differentially expressed genes when comparing naive B and GC-B cells between the mature and deviating TLSs.
On spatial transcriptomics data, deviating TLS and its proximal regions have significantly higher tryptophan scores than that of mature TLS. And maligment cells in TME have significantly higher scores among all cell types analyzed. To investigate the mechnism, the team further explored the epigenetic regulation of tryptophan-metabolizing enzymes (TDO2 and IDO2) in malignant cells using snRNA-seq and snATAC-seq. They found that chromatin accessibility at the promoter regions of tryptophan metabolism-related genes progressively decreased across malignant cell clusters, correlating with differences in metabolic activities. Therefore, metabolic reprogramming by tumour cells emerges as a local brake that diverts TLS from their natural maturation trajectory.
250905.png)
Figure 5A-B from Tang et al. (2025) [1] demonstrate that deviating TLS and their proximal region exhibit higher tryptophan signature scores compared to mature TLS.
To assess whether tryptophan metabolism affects TLS maturation in vivo, the team utilized an orthotopic HCC mouse model. They fed mice diets low, normal or high in tryptophan and then injected the tumors with CXCL13 + CCL21 to induce TLS. Researchers found that tryptophan restriction doubled mature TLS within two weeks versus high-tryptophan diets. The low-tryptophan group showed enhanced anti-PD-1 response with slower tumor growth. TDO2 overexpression or inhibition mirrored dietary effects, confirming tryptophan metabolism’s role in TLS maturation and ICB synergy. Thus, restriction of tryptophan metabolism can promote TLS maturation within TME and synergize with ICB to enhance anti-tumor immunity, revealing a promising strategy for boosting cancer immunotherapy.
250905.png)
Figure 6M-U from Tang et al. (2025) [1] shows the in vivo experimental design and the treatment outcomes, including tumor volume measurements and TLS frequency.
The study by Tang et al. (2025) advances our understanding of TLS biology in HCC by integrating spatial transcriptomics, single-cell multi-omics, and wet-lab experiments. Using the data-driven framework, the researchers uncovered the distinct developmental states of TLS and the role of tryptophan metabolism in restricting their maturation. The application of Stereo-seq technology provided a high-resolution spatial data that overcame the limitations of previous spatial transcriptomics tools, allowing for a comprehensive characterization of the tumor microenvironment. These insights not only elucidate mechanisms of immune evasion in HCC but also provide a translational roadmap to enhance immunotherapy efficacy in cancer. Future research will likely focus on further elucidating the mechanisms underlying TLS maturation and exploring the therapeutic potential of targeting tryptophan metabolism in cancer treatment.
STOmics now presents the upgraded Stereo-seq Transcriptomics Solution v1.3 launched in October 2024, featuring refined reagent chemistry, enhanced probe design, and optimized enzyme selection for whole transcriptome capturing of fresh frozen samples. This updated version delivers enhanced capture efficiency, broader compatibility, and a more streamlined workflow while minimizing the diffusion of mRNA molecules to achieve accurate spatial single-cell-level analysis. Explore more: https://en.stomics.tech/products/stereo-seq-transcriptomics-solution/list.html
1. Tang et al. Spatial transcriptomics reveals tryptophan metabolism restricting maturation of intratumoral tertiary lymphoid structures. Cancer Cell (2025) https://doi.org/10.1016/j.ccell.2025.03.011
2. Singal et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology (2023)https://doi.org/10.1097/HEP.0000000000000466
3. Teillaud et al. Tertiary lymphoid structures in anticancer immunity. Nat Rev Cancer (2024) https://doi.org/10.1038/s41568-024-00728-0
Author:
![]() Dr. Yahui Li, Field Application Scientist (Bioinformatics) at STOmics Tech
Dr. Yahui Li, Field Application Scientist (Bioinformatics) at STOmics Tech