All
Products
Resources
News
FAQ
Search
14/01/2025 Xin LIU, Jiajun ZHANG
The tumor microenvironment (TME) constitutes a highly intricate ecosystem composed of various cell populations, including tumor cells and non-tumor cells. The composition, intercellular interactions and functional status of the cells associated with the process of tumor development, invasion and metastasis, and reshape the TME to respond to therapeutic interventions. The dynamic alterations in the cellular components and their functions within the ecosystem contribute to the pronounced inter- and intra-tumor heterogeneity, significantly magnifying the challenges in disease management and the development of anti-cancer drugs. Consequently, a profound understanding of the intricate interactions between tumor cells and stromal cells is essential for the development of effective anti-cancer therapies.
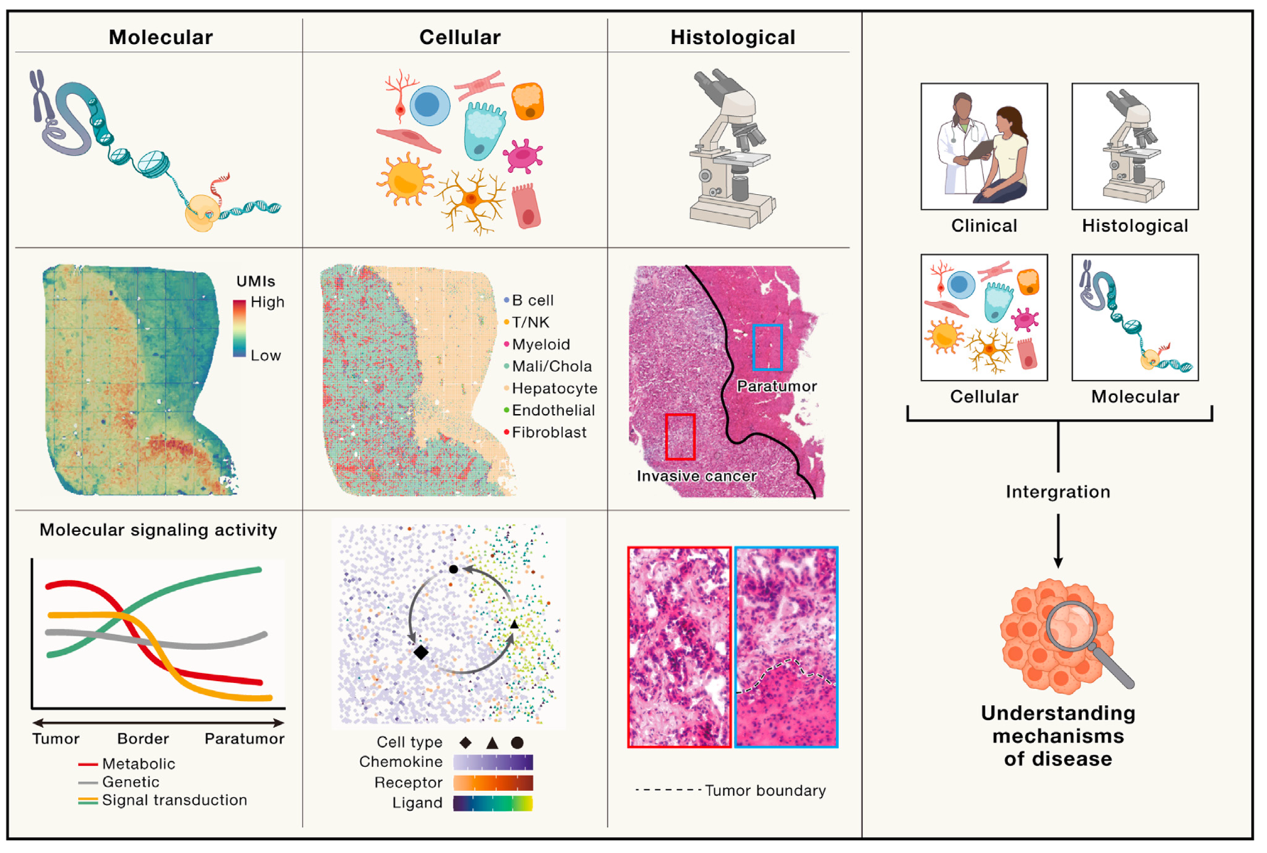
Fig.1 Summary of how spatial omics uncover pathogenesis of diseases [1]
STOmics is the pioneer of state-of-the-art spatially-resolved multi-omics technologies with the DNB-based Stereo-seq technology. STOmics provides the world's leading spatiotemporal multi-omics product set, which enables unbiased discoveries at both the protein and transcriptome levels within the same tissue section at single-cell resolution. This capability addresses biological questions across scientific and translational research. The technology offers a comprehensive analysis of the tumor microenvironment from a multi-modal perspective. It not only reveals the cellular composition but also provides insights into distinct cellular functions and intercellular communication. This technology empowers scientists to inverstigate the tumor microenvironment with promising accuracy, providing the spatial context missing in techonologies, like single-cell sequencing.
Efficacy and toxicity are the major challenges for success of drug development. Gathering evidence of mode of action and resistance mechanims during preclinical and clinical development will benefit the development process by removing uncertainties and reducing time and cost. By perfoming Stereo-seq analysis on tissues collected before and after treatment, scientists can vividly map the dynamic remodeling of the diseased tissue microenvironment in response to drugs of interest. This approach allows scientists for the identification of spatially defined functional subunits, which can significantly enhance researchers' knwoledge of the pharmacological activities and drug resistance mechanisms. Consequently, it solidify mechanistic evidence for the clinical application of novel drug therapies. (Fig 2)
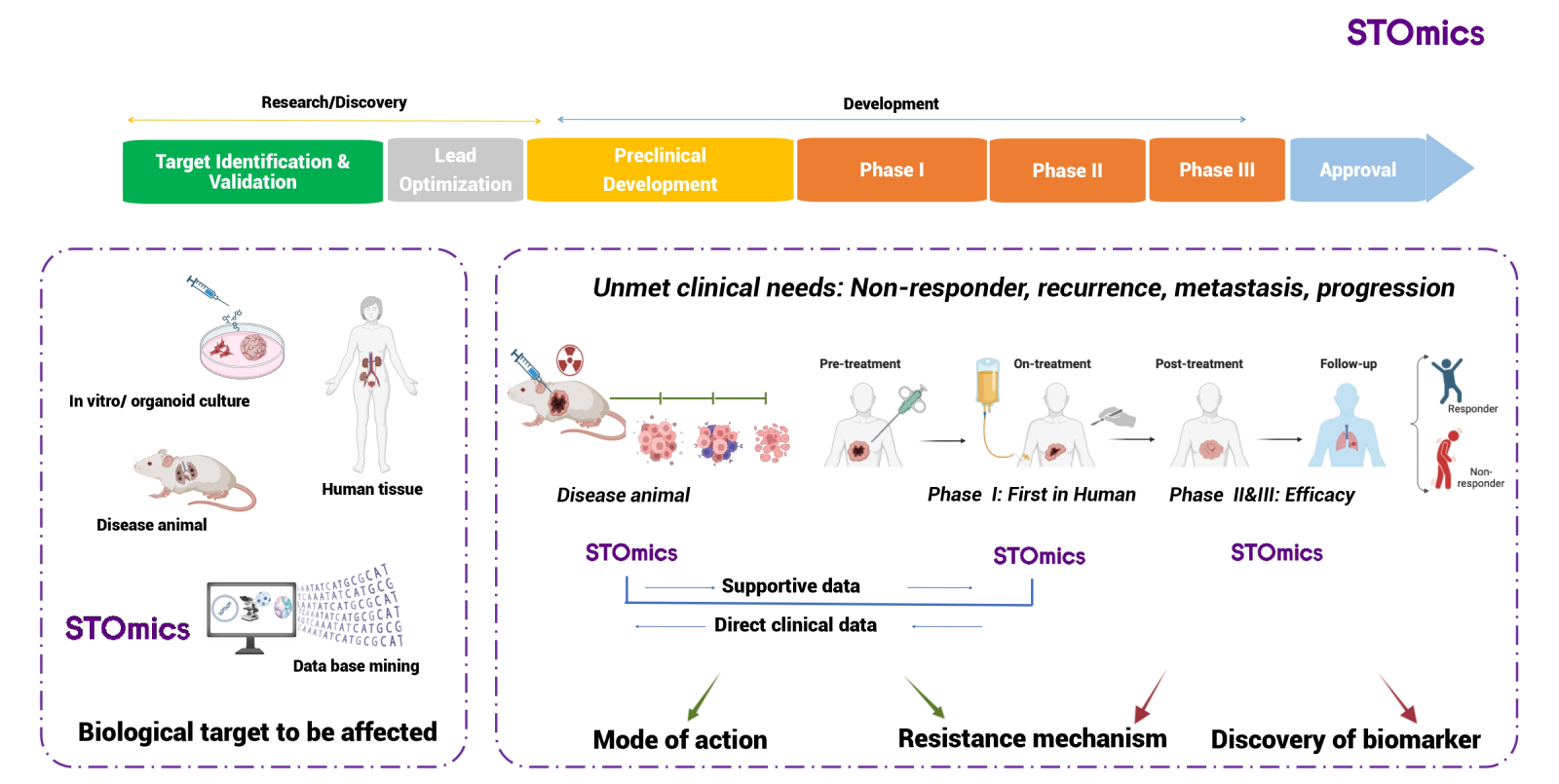
Fig 2. Implementation of stereo-seq omics in drug R&D process: a scientific foundation for the development of drugs
In a preclinical study involving xenografted mice treated with STING (a stimulator of interferon genes) agonist, as tumor samples detected by Stereo-seq, researchers identified a unique cellular neighborhood (CN) that correlates with the tumor's response to STING. Within this CN, the IL1b-IL1R1 and CXCL2-CXCR2 signaling pathways were found to be spatially co-localized, and the STING agonist-associated signaling pathways, including NF-κB and IRF1 transcriptional regulation, were activated. These discoveries elucidate the mechanism by which STING agonists exert their effects in tumor treatment and uncover potential therapeutic targets for cancer treatment (Fig 3). [2]. In another preclinical study,the application of IFNα-LV for the treatment of colorectal cancer with liver metastases was investigated. Spatiotemporal omics analysis unveiled the differential response of various regions within the metastatic lesions to the drug treatment. Functional analysis elucidated the drug's mechanism of action and identified IL-10-mediated immunosuppression as a potential mechanism underlying drug resistance.
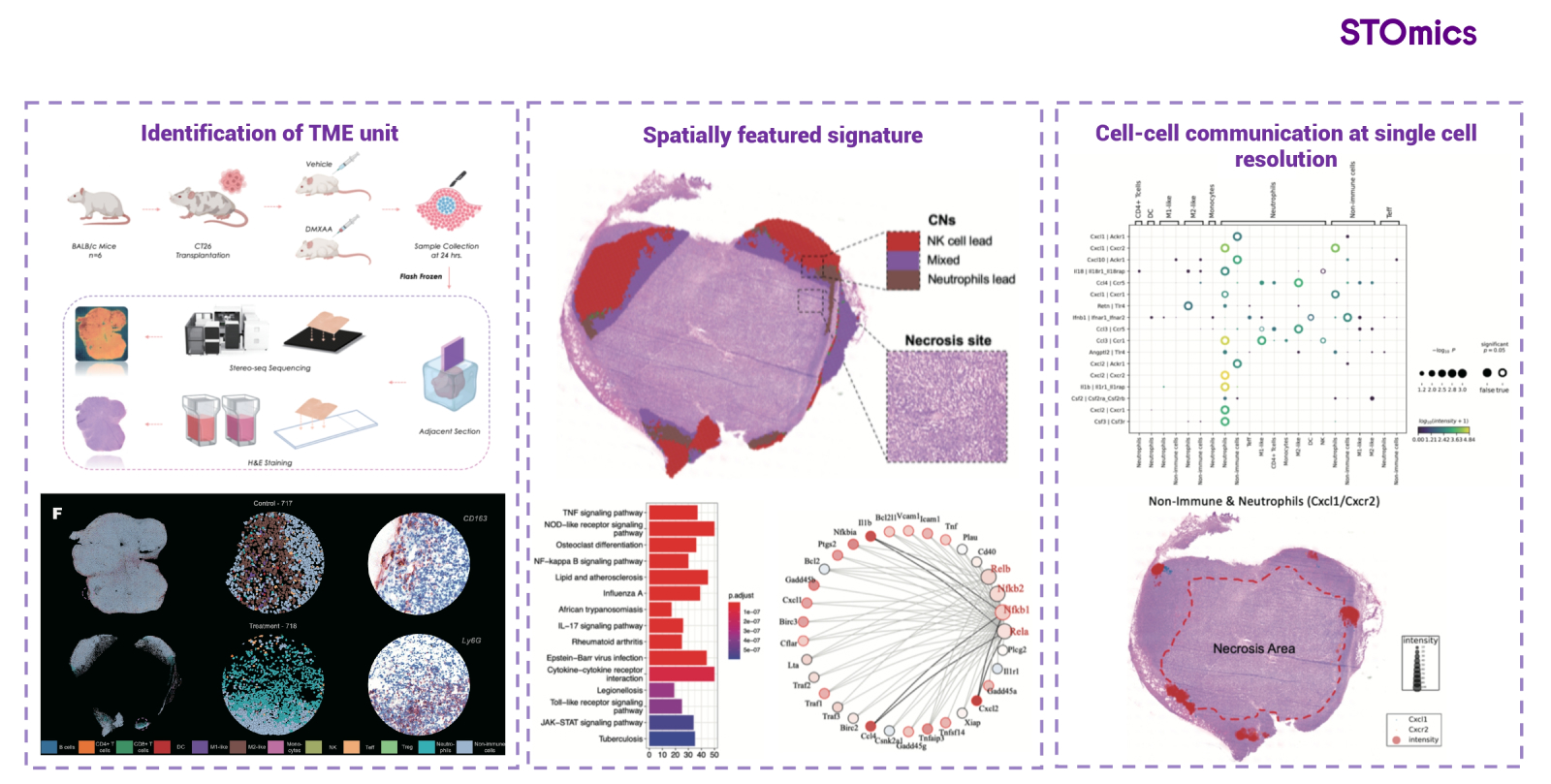
Fig 3. Exploration of drugable targets & of important mechanisms by Stereo-seq
Beyond animal models, spatiotemporal omics technology has been a useful tool in early-phase clinical studies of drug therapy, offering novel insights into the mechanisms of drug action with samples from patients. The anti-netrin-1 antibody (NP137) represents a pioneering approach to targeting netrin-1 in advanced endometrial carcinomas. In the phase I clinical study of NP137, spatial transcriptome profiling revealed that this treatment can reverse the epithelial-mesenchymal transition (EMT) in endometrial cancer cells and enhance the interaction between CD8+ T cells and tumor cells, leading to tumor regression in patients [4,5]. The results suggest that NP137 is able to potentially sensitize cancer cells to chemotherapy, which guide the following comination treatment strategy of clinical trial.
Immune checkpoint inhibitors (ICIs) exhibit limited clinical efficacy in patients with advanced soft-tissue sarcomas (STSs), with the exception of those with positive intratumoral tertiary lymphoid structures (TLSs). The phase II PEMBROSARC trial demonstrated a 6-month non-progression rate (NPR) of 40% and an objective response rate (ORR) of 30% in TLS-positive STS patients. In contrast, the all-comer cohorts showed a significantly lower 6-month NPR of 4.9% (95% CI, 0.6-16.5) and an ORR of 2.4% (95% CI, 0.1-12.9). Integrated analysis of spatial transcriptome data with protein staining indicated that the presence of intratumoral plasma cells was significantly associated with improved outcomes, while the abundance of Treg cells had a negative impact [6]. It sets up the foundation for development of novel biomarkers for patient stratification.
As previously discussed, the intricate tumor microenvironment is subject to constant flux, and the limitations of previous technologies in terms of low throughput and absence of spatial context have significantly impeded more precise and profound investigations into tumors. The emergence of spatiotemporal omics technology has allowed many researchers to meticulously dissect the tumor microenvironment, yielding deeper insights into the dynamics of the tumor microenvironment throughout tumorigenesis and disease progression.
Employing an invasive tumor border scanning and digitization model facilitated by Stereo-seq, Wu et al. defined a 500 µm-wide "invasive zone" surrounding the tumor border in liver cancer patients. This zone was characterized by immunosuppression, metabolic reprogramming, and severely compromised hepatocytes. Furthermore, they identified a subpopulation of damaged hepatocytes with elevated expression of serum amyloid A1 and A2 (collectively known as SAAs), located in close proximity to the tumor border on the paratumor side. These cells were found to attract macrophages, induce M2 polarization, and further exacerbate local immunosuppression, potentially contributing to tumor progression . The discovery of this novel invasive zone in cancer patients not only enhances our understanding of tumor invasion and metastasis but also suggests new avenues for potential anti-tumor therapeutic strategies.
In recent years, the focus on tumor-associated fibroblasts (CAFs) has intensified, recognizing their pivotal role in anti-tumor immunity, tumor progression, and migration. Spatiotemporal omics analysis, with its capacity to study cells that exert bidirectional effects and engage in complex interactions, has proven particularly advantageous in this area of research. Utilizing stereo-seq technology, Chen et al. identified a myofibroblastic CAF subpopulation, POSTN+ CAFs, in non-small-cell lung cancer (NSCLC) patients. This subpopulation may collaborate with SPP1+ macrophages to foster the development of desmoplastic architecture and contribute to immune suppression. Moreover, they demonstrated that POSTN+ CAFs are linked to cancer progression and poor clinical outcomes, offering fresh perspectives on NSCLC treatment strategies [8].
In a separate study, researchers identified a cluster of pro-tumorigenic cancer-associated myofibroblasts (myCAFs) in advanced cervical squamous cell carcinoma (CSCC). MyCAFs are believed to bolster tumor growth and metastasis by curbing lymphocyte infiltration and reshaping the tumor's extracellular matrix. These myCAFs are also correlated with a reduced survival probability in CSCC patients, highlighting their significance in disease prognosis and potential therapeutic targeting [9].
STOmics dedicates to lead the implementation of Stereo-seq workflow to drug development and clinical translation to improve the outcome of patients. The Stereo-seq technology offers impetus to solve unmet needs in clinic to improve quality of life in real world (Fig 4).
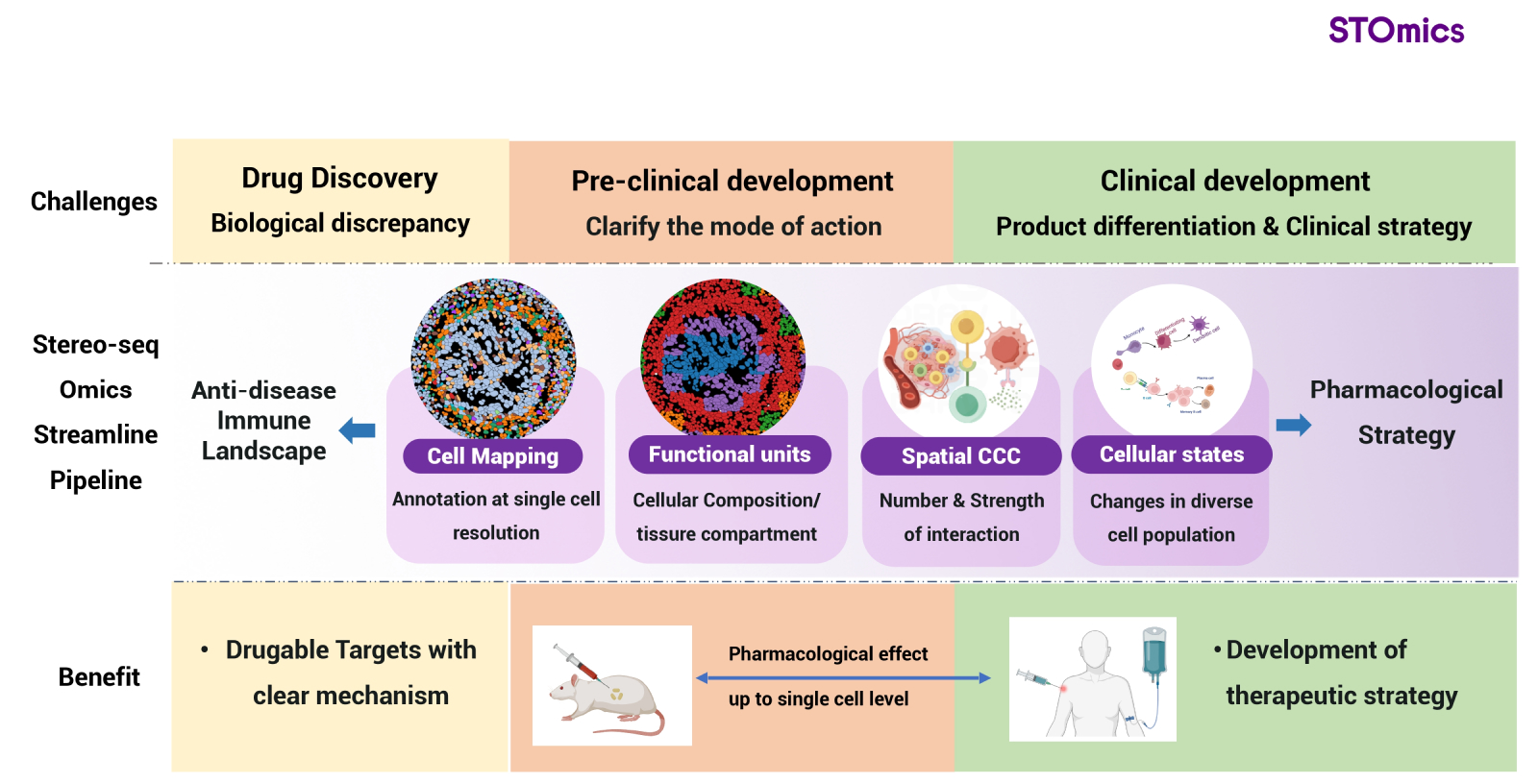
Fig 4. Stereo-seq workflow: next generation of drug development and clinical application
1. Liu L et al. Spatiotemporal omics for biology and medicine. Cell. 2024 Aug 22.
2. Xing Liu et al. StereoSiTE: A framework to spatially and quantitatively profile the cellular neighborhood organized iTME. bioRxiv 2022.12.31.522366; doi: https://doi.org/10.1101/2022.12.31.522366
3. Kerzel T et al, In vivo macrophage engineering reshapes the tumor microenvironment leading to eradication of liver metastases. Cancer Cell. 2023 Nov 13;41(11):1892-1910.e10. doi: 10.1016/j.ccell.2023.09.014. Epub 2023 Oct 19. PMID: 37863068.
4. Lengrand J et al., Pharmacological targeting of netrin-1 inhibits EMT in cancer. Nature. 2023 Aug;620(7973):402-408. doi: 10.1038/s41586-023-06372-2. Epub 2023 Aug 2. PMID: 37532929; PMCID: PMC7615210.
5. Cassier PA et al., Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature. 2023 Aug;620(7973):409-416. doi: 10.1038/s41586-023-06367-z. Epub 2023 Aug 2. PMID: 37532934; PMCID: PMC10412451.
6. Italiano A et al, Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat Med. 2022 Jun;28(6):1199-1206. doi: 10.1038/s41591-022-01821-3. Epub 2022 May 26. PMID: 35618839.
7. Wu L et al., An invasive zone in human liver cancer identified by Stereo-seq promotes hepatocyte-tumor cell crosstalk, local immunosuppression and tumor progression. Cell Res. 2023 Aug;33(8):585-603. doi: 10.1038/s41422-023-00831-1. Epub 2023 Jun 19. PMID: 37337030; PMCID: PMC10397313.
8. Chen C et al., Single-cell and spatial transcriptomics reveal POSTN+ cancer-associated fibroblasts correlated with immune suppression and tumour progression in non-small cell lung cancer. Clin Transl Med. 2023 Dec;13(12):e1515. doi: 10.1002/ctm2.1515. PMID: 38115703; PMCID: PMC10731139.
9. Ou Z et al,Single-Nucleus RNA Sequencing and Spatial Transcriptomics Reveal the Immunological Microenvironment of Cervical Squamous Cell Carcinoma. Adv Sci (Weinh). 2022 Oct;9(29):e2203040. doi: 10.1002/advs.202203040. Epub 2022 Aug 19. PMID: 35986392; PMCID: PMC9561780.